Full Text View
Volume 22 Issue 1 (January 2012)
GSA Today
Article, pp. 4-9 | Abstract | PDF (2.2MB)
Speciation collapse and invasive species dynamics during the Late Devonian “Mass Extinction”
| Table of Contents |
|---|
|
Search GoogleScholar for
Search GSA Today |
Abstract
The Late Devonian (Frasnian-Famennian) interval includes one of the most dramatic intervals of biotic turnover in the Phanerozoic. Statistical evaluation of diversity change reveals that the primary cause of biodiversity decline was reduced speciation during the crisis interval, not elevated extinction rates. Although various hypotheses have been proposed to explain extinction increase during the Late Devonian, potential causes for reduced speciation have previously been largely unaddressed. Recent analyses focusing on biogeographic and phylogenetic patterns of species in shallow marine ecosystems of Laurentia indicate that a dramatic increase in interbasinal species invasions, facilitated by transgressive pulses, fundamentally affected biodiversity by enabling range expansion of ecological generalists and eliminating vicariance, the primary pathway by which new species typically form. Modern species invasions may result in similar speciation loss, exacerbating the current biodiversity crisis.
*E-mails:
Manuscript received 18 May 2011; accepted 3 Nov. 2011.
DOI: 10.1130/G128A.1
Introduction
A dramatic interval of biodiversity loss and ecosystem reorganization occurred at the boundary between the Frasnian and Famennian stages of the Late Devonian Period (ca. 375 Ma). This event was originally considered to rank among the “Big Five” mass extinction events in the Phanerozoic (Raup and Sepkoski, 1982), and it is still listed as the “Frasnian-Famennian Mass Extinction” in most introductory and historical geology textbooks. The designation of “mass extinction,” however, is misleading because the Frasnian extinction rate was neither elevated relative to the Middle Devonian nor statistically higher than the background rate of extinction throughout the Phanerozoic (Bambach et al., 2004; Alroy, 2008). Rather, an anomalously low rate of speciation, the origination of new species, was the primary cause of this decline in biodiversity (Bambach et al., 2004).
Global standing biodiversity is controlled equally by the number of new species forming and the number of species becoming extinct during an interval. All episodes of biodiversity loss require that extinction rate exceeds speciation rate. For an event to be classified as bona fide mass extinction, however, the extinction rate of the crisis interval must statistically exceed both the background extinction rate of the Phanerozoic and be elevated above that of the adjacent stages. Biodiversity crises occur when speciation rates have a statistically significant decline compared to the background rate while extinction rates remain within the limits of statistical normal. Reduced speciation rate combined with slightly elevated extinction levels can result in a dramatic biodiversity crisis, and this is what transpired during the Late Devonian. The Frasnian-Famennian event is, therefore, better termed a “biodiversity crisis” than a “mass extinction.”
The shift in status of the Frasnian-Famennian event from a “mass extinction” to a “biodiversity crisis” does not imply a reduction in the severity of the effects on global ecosystems. In fact, the level of marine ecosystem reorganization that occurred during the Late Devonian, including a fundamental collapse of the reef ecosystem, is second only to the Permo-Triassic mass extinction (McGhee et al., 2004). The Middle Devonian included the most geographically widespread metazoan reef ecosystem in Earth’s history, but its extent was reduced by a factor of 5000 following the crisis interval (Copper, 1994). Other biotic changes included the spread of cosmopolitan species facilitated by rampant species invasions documented across many clades (reviewed in McGhee, 1996).
A series of local and global environmental changes occurred coincident with biotic overturn. These included changes related to the development of complex forest ecosystems on land, such as eutrophication and alteration of terrestrial weathering patterns (Algeo and Scheckler, 1998), high frequency sea-level changes (ver Straeten et al., 2011), widespread anoxia events (Buggisch and Joachimski, 2006), overall warming of the global oceans (van Geldern et al., 2006), and pulses of enhanced carbon burial that resulted in rapid cooling events at the Frasnian-Famennian boundary (van Geldern et al., 2006; also see reviews in McGhee, 1996, 2005; Racki, 2005). Most of these environmental factors (and various combinations of them) have been proposed as drivers for the “mass extinction.” Theoretically, abrupt or even gradual changes in environmental conditions could result in increased extinction of species because extinction occurs when members of a species can no longer cope with changing environmental conditions (abiotic or biotic) and population size decreases to zero. These environmental factors are undoubtedly involved with ecosystem degradation and certainly contributed to the observed elevation of extinction levels.
None of these abiotic changes, however, supply a satisfactory explanation for speciation collapse because they do not directly impact the speciation process. In order to better understand the ecological crisis during the Late Devonian, the mechanisms of speciation decline must be examined. A speciation event is a unique episode in geologic time that transpired at a discrete geographic location within a specific lineage of organisms. Identifying causal factors for speciation decline requires both a detailed temporal and geographic framework and robust hypotheses of ancestor-descendant relationships (Benton and Pearson, 2001). This type of detail is only available in clades for which species-level phylogenetic hypotheses have been generated. Therefore, speciation analysis requires a fundamentally different and more detailed dataset than analyses of potential extinction mechanisms. Although the significance of speciation reduction during this interval has been known for more than 25 years (McGhee, 1984), sufficiently resolved phylogenetic data have only recently become available to assess changes in speciation mode across the Middle to Late Devonian interval. Significantly, recent analyses discussed herein indicate that the widespread extra-basinal migrations of species (analogous to modern invasive species) during the Late Devonian facilitated speciation decline by preventing geographic isolation, the primary process by which new species arise.
Origination and Speciation Rate Analyses
The importance of reduced origination in driving Frasnian diversity decline was first recognized by McGhee (1984) from analyses of genera of articulate brachiopods from Catskill delta complex of eastern North America and from the Ural Mountains. Subsequent analyses (e.g., Foote, 1994; Bambach et al., 2004; Alroy, 2008) using stratigraphic range data for marine invertebrate families and genera culled from global compendia confirmed that reduced origination was the primary driver of biodiversity collapse (Fig. 1A). Certainly, patterns of biodiversity change were not congruent across all clades. Some previously prolific clades, such as the atrypid brachiopods, experienced high extinction rates, while other clades, including the crinoids, radiated to effect a pronounced change in post-crisis shallow marine ecosystems (see discussion in Racki, 2005). However, the general pattern of depressed origination—but only moderately elevated extinction rates—documented in cross-faunal database analyses is robust to variations in sampling procedures, rate metric used, taxonomic level (family vs. genus) analyzed, or database employed (Foote, 1994; Bambach et al., 2004; Alroy, 2008). This supports depressed origination as a primary driver of Late Devonian biodiversity loss.
|
Comparison of extinction versus origination/speciation across the Middle to Late Devonian interval. Late Devonian Biodiversity Crisis interval is indicated in yellow. (A) Proportion of generic extinction or origination per interval. Modified from Bambach et al. (2004). (B) Instantaneous rates of species extinction and speciation for two brachiopod genera (Schizophoria and Floweria), one bivalve genus (Leiopteria), and all three clades combined. Modified from Stigall (2010a). Similar patterns occur at both taxonomic levels: Origination/speciation rates are substantially reduced during the crisis interval, but extinction rates are lower than during the Middle Devonian background interval. |
From a biological standpoint, the most appropriate taxonomic level to assess reduced origination is the species level. Species are biological entities that are defined by attributes related to reproductive cohesion in both time and space (deQueiroz, 2007). Therefore, analysis of origination at the species level equates to examination of actual biological processes, whereas generic and familial analyses are increasingly distant proxies. Species-level phylogenetic hypotheses, which include an evolutionary framework to constrain timing of speciation events, are necessary to calculate the most accurate speciation rates (Smith, 1994). Unfortunately, very few species-level phylogenies have been published with substage temporal resolution for Late Devonian clades (e.g., Rode, 2004; Stigall Rode, 2005).
Stigall (2010a) utilized recently published species-level phylogenetic hypotheses of Rode (2004) and Stigall Rode (2005) for three Late Devonian clades (two articulated brachiopod genera and one bivalve subgenus), primarily from North America, to examine whether reduced origination was also significant at the species level. These clades serve as a reasonable proxy for shallow marine biota of Laurentia because these monophyletic lineages had excellent preservation potential, include common members of the shallow marine benthos, and their combined fifty species inhabited the full suite of nearshore to offshore marine environments. Results are consistent with the earlier analyses based on higher taxa (Fig. 1B). Overall biodiversity plummeted during the Frasnian crisis interval, and this change was driven primarily by speciation loss (Stigall, 2010a). Late Frasnian extinction rates, while moderately elevated, do not exceed pre-crisis levels for any clade and are not statistically higher during the crisis interval than the average value for each rate over the duration of the clade (Stigall, 2010a).
The combination of species, generic, and family-level analyses firmly establishes the loss of speciation as a fundamental driver for biodiversity loss during the Late Devonian, at least among shallow marine taxa where the crisis was most pronounced. Examining the process of speciation and the factors that promote or hinder that process is, therefore, required to identify causal factors for the crisis.
Speciation Mode Analysis
Investigating the cause of speciation collapse during the Late Devonian first requires determining which speciation mechanisms were compromised during the crisis interval. Speciation requires a group of organisms to become reproductively isolated from its ancestral population in order to establish a new biological entity. This isolation typically occurs via either geographic separation of the incipient species from the ancestral population (allopatric speciation) or via shifts in reproductive timing or chromosomal count within the same geographic space as the ancestor (sympatric speciation) (Mayr, 1963). Sympatric speciation is commonly undetectable in the fossil record, but allopatric speciation is perceptible because it typically results in morphological shifts as incipient species adapt to environmental conditions that differ from those of the ancestral range. Allopatric speciation occurs via two primary mechanisms: vicariance and dispersal, which are characterized by discrete biogeographic patterns related to the geographic range of daughter species relative to the ancestral population (Fig. 2) (Wiley and Mayden, 1985). Thus, it is possible to identify speciation events of each type in fossil taxa where evolutionary relationships are known and ancestral ranges can be inferred (Lieberman, 2000) (Fig. 3).
|
Geography of allopatric speciation modes. In vicariance, the ancestral population (Species A) is passively divided by a geographic barrier. Incipient species (Species A' and A") form during geographic isolation and later diverge to become new species (Species B and C). In dispersal, a subpopulation of the ancestral species (Species A) actively migrates across a geographic barrier to form an incipient species (Species A'), which later diverges to become a new species (Species B). |
|
Biogeographic areas mapped onto a strato-cladogram for the brachiopod genus Floweria. Speciation events where daughter species occupy a subset of the ancestral range are interpreted as vicariance events. Speciation events where daughter species occupy areas additional to the ancestral areas are interpreted as dispersal events. Modified from Stigall (2010a). |
To assess speciation mode during the Late Devonian, Stigall (2010a) conducted a biogeographic analysis on species-level phylogenetic hypotheses of four common groups of Devonian marine organisms: an order of predatory crustaceans, one bivalve genus, and two brachiopod genera, published in Rode and Lieberman (2002), Rode (2004), and Stigall Rode (2005), respectively. This cross-phyla analysis included common taxa within both the sessile benthos and pelagic predator guilds and, thus, is a reasonable proxy for faunal dynamics in shallow marine environments of Laurentia. Speciation by vicariance was limited relative to speciation by dispersal in each of these clades, ranging from only 12% to 50% of quantifiable speciation events, for a combined rate of 28% speciation by vicariance versus 72% speciation by dispersal. Similar analyses of the modern biota have demonstrated vicariance to be the dominant form of speciation by a factor of almost 3 to 1 (Brooks and McLennan, 2002), and analyses of speciation mode conducted for other Paleozoic intervals (reviewed in Stigall, 2010a) have always recovered higher frequencies of speciation by vicariance versus dispersal (Table 1).
|
Comparison of speciation mode through geologic time |
Speciation mode during the Late Devonian is evidently different from the typical pattern in Earth history. This incongruity provides the framework for a mechanistic explanation for speciation decline during the crisis interval. During the Late Devonian, vicariance, normally the most prevalent style of speciation, was essentially extinguished. In fact, each of the few vicariance events present in the clades analyzed precede the late Frasnian crisis interval (Stigall, 2010a). Speciation by dispersal, although still operational during the crisis interval, typically occurred at a lower rate and accordingly resulted in few Late Devonian speciation events. Therefore, elimination of the dominant mode of speciation led to the dramatic reduction in speciation rate, and consequently biodiversity, at the end of the Frasnian.
The differential loss of speciation type provides a foundation against which to analyze causes of biodiversity decline. Satisfactory causes for biodiversity collapse must be able to explain both the lack of vicariant speciation and the slight elevation in extinction rates. Abiotic explanations alone, such as global cooling or basin anoxia, do not provide adequate explanations for the differential reduction in vicariance compared to speciation by dispersal; however, biotic factors, such as the spread of invasive species, potentially could.
Invasive Species During the Late Devonian
Extensive interbasinal species migrations have been documented in many clades during the Frasnian (reviewed in McGhee, 1996). These migrations are characterized by the dispersal of a species native to one tectonic basin into a second tectonic basin outside its original geographic range (Fig. 4). Because these species establish secondary populations in ecosystems in which they did not evolve, they are analogous to modern invasive species (Vermeij, 2005; Stigall, 2010b). Species migrations have occurred throughout geologic time; however, most episodes of biotic exchange are limited to a localized dispersal pathway (e.g., Great American Biotic Interchange; see review in Vermeij, 2005). During the Late Devonian, species introductions were rampant on a global scale.
|
Interbasinal invasions of Late Devonian brachiopod, Pseudatrypa devoniana. (A) During the early Frasnian Stage, P. devoniana occupied its ancestral basin in the New York region. (B) This species invaded the Iowa basin during the middle Frasnian and (C) subsequently invaded the New Mexico basin during the late Frasnian. Invasions correspond to sea-level rises indicated by the second and third arrow in Figure 5. Modified from Rode and Lieberman (2004). |
The impact of these Late Devonian invasions was quantified by Rode and Lieberman (2004) using Geographic Information Systems–based analyses to calculate geographic ranges and map invasion events in more than 300 Middle and Late Devonian articulate brachiopod and bivalve species of Laurentia. Species ranges were mapped at conodont zone resolution to produce a high-precision temporal framework for identifying invasion events (Fig. 5). A substantial increase in invasion intensity occurred coincident with the decline in speciation during the Frasnian; 68% of all identified invasion events occurred in the Frasnian. Rapid transgressions provided pathways for species dispersal; 65% of Frasnian invasion events correlate with transgressive events. Additionally, the mean size of both native and invasive species’ geographic ranges increased during the Frasnian (Rode and Lieberman, 2004). Moreover, species with larger geographic ranges, an episode of interbasinal invasion in their history, and/or expansion of their geographic ranges during the late Frasnian survived into the Famennian at statistically higher rates than non-invasive species with narrow geographic ranges (Rode and Lieberman, 2004; Stigall Rode and Lieberman, 2005).
|
Interbasinal invasion intensity during the Middle and Late Devonian. The spread of invasive species was facilitated by sea-level rises, indicated by blue arrows. Modified from Rode and Lieberman (2004). |
These species invasions, facilitated by sea-level changes, could have caused the observed reduction in speciation coupled with moderately elevated extinction. The combination of overall range expansion and frequent invasive events would have prohibited sustained geographic isolation, thereby impeding the primary requirement for vicariant speciation, as well as hindered the successful development of migrant populations into new species, thus restricting speciation by dispersal. The preferential extinction of species with small geographic ranges could have produced the observed elevation of extinction levels.
Synthesizing Invasive Species Effects, Ecology, and Speciation
The results of speciation and biogeographic analyses provide a framework in which to examine the mechanisms that reduced speciation and slightly elevated extinction rates during the Late Devonian Biodiversity Crisis. In particular, three features—differential extinction of narrowly ranging species, impact of invaders on native species, and macroevolutionary differences between ecological generalist and specialist species—are critical for explaining biodiversity decline.
A striking feature of the Late Devonian biogeographic pattern is the differential survival of species with large geographic ranges. Species with larger geographic ranges tend, on average, to have broader ecological tolerances than those with small ranges (Jackson, 1974; Fernández and Vrba, 2005). Ecological specialists are confined in terms of both their habitat preferences and the geographic region where those conditions occur (Stanley, 1979). Conversely, ecological generalists can successfully utilize a wider set of environmental conditions, which typically allows them to occupy larger geographic areas. Thus, although Middle Devonian biotas included both ecological specialists and generalists, most native species that survived the crisis had large geographic ranges (Rode and Lieberman, 2004) and were presumably ecological generalists.
Furthermore, Devonian invaders were dominantly, if not exclusively, ecological generalists. Modern invasive species are characterized by broad environmental tolerances, which contribute to their ability to survive during both the transport and establishment phases of invasion (Lockwood et al., 2007). Devonian invaders were likely similar, because ecological niches of Devonian invaders must have been sufficiently broad to allow colonization of both the invasion pathway and the new tectonic basin. Consequently, the arrival of the Devonian invaders into new tectonic basins effectively resulted in an influx of new ecological generalists into the ecosystem. Studies of modern and Cenozoic invasive species have demonstrated that invader species regularly displace native species through higher resource efficiency (Johansson, 2007) or competitive ability (Vermeij, 2005). Similar processes operating during the Late Devonian would have caused differential extinction of narrowly ranging ecological specialist species. This resulted in elevated extinction rates and a proportional increase of broad ranging ecological generalists versus geographically restricted specialists in the biota.
Clades of ecological generalists tend to have lower speciation rates and contain fewer species relative to specialist lineages (Vrba, 1987; Eldredge, 1989). This discrepancy relates to the mechanics of the allopatric speciation process. If a group of specialists undergoes vicariance, it will likely be exposed to environmental conditions that differ in some way from their ancestral range, and the population must either adapt to those conditions or become extinct. On the other hand, generalists are more likely to be pre-adapted via their broad ecological niche to the new set of conditions encountered so that no adaptive change is required. Consequently, specialist lineages experience both higher speciation and extinction rates than ecological generalists.
The differential extinction of native specialist species during the Late Devonian reduced the potential ancestral species pool from which new specialist species could evolve, which resulted in speciation depression. Furthermore, native and invasive generalist lineages would have had few opportunities to speciate as expansion of geographic ranges facilitated by sea-level rise prevented effective long-term vicariance from ancestral populations. Rather, incipient generalist species were more likely to be subsumed as a geographic extension of the expanding ancestral species than to develop into new species. This combination of preferential extinction of specialist species and expansion of the geographic ranges of generalist species (native and invasive) facilitated the dramatic speciation reduction of the Late Devonian.
This pattern of differential survival, range expansion, and speciation occurred within the most common components of the Late Devonian shallow marine ecosystem but may not be transferable to all marine clades or other environments. Central to this argument is the frequency of range expansion among native generalist species and the introduction of invaders resulting in competitive interactions on the seafloor. Continental ecosystems, including both terrestrial and freshwater habitats, and marine taxa potentially less amenable to these processes did not experience the same level of biodiversity loss during the Late Devonian (reviewed in McGhee, 1996).
Conclusions
The Late Devonian Biodiversity Crisis was one of the most significant intervals of biodiversity loss and faunal overturn during the Phanerozoic. Unlike “true” mass-extinction events, such as the Late Permian and End Cretaceous events, the primary driver of biodiversity loss was a severe reduction in speciation rate, not substantially elevated levels of extinction. Purely abiotic explanations for biodiversity loss during the Late Devonian fail to provide a complete explanation for the biodiversity crisis. Shifts in Late Devonian biogeographic patterns were driven by range expansion of generalist taxa within basins and rampant species invasions between basins associated with transgressive events. These shifts provide a mechanistic explanation for the reduction in speciation during the crisis interval, particularly speciation by vicariance.
The central role of invasive species in mediating biodiversity decline during that Late Devonian Biodiversity Crisis parallels aspects of modern biodiversity crisis affecting our planet. The primary drivers of the current biodiversity crisis are habitat destruction, climate change, and the spread of invasive species (Thuiller, 2007). The current rate of biodiversity loss is as high as or higher than during any interval in the Phanerozoic (Barnosky et al., 2011). The impacts of habitat degradation and climatic change have long been analyzed within the context of geologic time and are known to cause substantial elevation of extinction rates. Comparison with the Late Devonian interval suggests that the modern influx of invasive species will result in substantially reduced speciation rates. The modern combination of habitat destruction coupled with species introductions is, therefore, likely to result in total biodiversity loss that may be even greater than that experienced during the Late Permian coupled with an extensive recovery interval due to speciation depression. These implications highlight the need for conservation efforts to target specialist taxa for protection in addition to preventing species introductions and preserving habitat.
Acknowledgments
Thanks to Peter Harries, Bernard Housen, and three anonymous reviewers for constructive reviews and to Damian Nance for the invitation to submit this paper to GSA Today. This research was supported by U.S. National Science Foundation grant EAR-0922067 and a grant from donors to the American Chemical Society’s Petroleum Research Fund.
REFERENCES CITED
- Algeo, T.J., and Scheckler, S.E., 1998, Terrestrial-marine teleconnections in the Devonian: Links between the evolution of land plants, weathering processes and anoxic events: Philosophical Transactions of the Royal Society of London, Series B, v. 353, p. 113–130.
- Alroy, J., 2008, Dynamics of origination and extinction in the marine fossil record: Proceedings of the National Academy of Sciences, v. 105, p. 11,536–11,542.
- Bambach, R.K., Knoll, A.J., and Wang, S.C., 2004, Origination, extinction, and mass depletions of marine diversity: Paleobiology, v. 30, p. 522–542.
- Barnosky, A.D., Matzke, N., Tomiya, S., Wogan, G.O.U., Swartz, B., Quental, T., Marshall, C., McGuire, J.L., Lindsey, E.L., Maguire, K.C., Mersey, B., and Ferrer, E.A., 2011, Has the Earth’s sixth mass extinction already arrived?: Nature, v. 471, p. 51–57.
- Benton, M.J., and Pearson, P.N., 2001, Speciation in the fossil record: Trends in Ecology and Evolution, v. 16, p. 405–411.
- Brooks, D.R., and McLennan, D.A., 2002, The Nature of Diversity: Chicago, University of Chicago Press, 668 p.
- Buggisch, W., and Joachimski, M.M., 2006, Carbon isotope stratigraphy of the Devonian of Central and Southern Europe: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 240, p. 68–88.
- Copper, P., 1994, Ancient reef ecosystem expansion and collapse: Coral Reefs, v. 13, p. 3–11.
- deQueiroz, K., 2007, Species concepts and species delimitation: Systematic Biology, v. 56, p. 879–886.
- Eldredge, N., 1989, Macroevolutionary Dynamics: Species, Niches, and Adaptive Peaks: New York, McGraw-Hill Publishers, 226 p.
- Fernández, M.H., and Vrba, E.S., 2005, Body size, biomic specialization and range size of large African mammals: Journal of Biogeography, v. 32, p. 1243–1256.
- Foote, M., 1994, Temporal variation in extinction risk and temporal scaling of extinction metrics: Paleobiology, v. 20, p. 424–444.
- Jackson, J.B.C., 1974, Biogeographic consequences of eurytopy and stenotyopy among marine bivalves and their evolutionary significance: The American Naturalist, v. 108, p. 541–560.
- Johansson, J., 2007, Evolutionary responses to environmental changes: How does competition affect adaptation? Evolution, v. 62, p. 421–435.
- Lieberman, B.S., 2000, Paleobiogeography: Using Fossils to Study Global Change, Plate Tectonics, and Evolution: New York, Kluwer Academy/Plenum Publishing, 208 p.
- Lockwood, J., Hoopes, M., and Marchetti, M., 2007, Invasion Ecology: Singapore, Wiley-Blackwell Publishing, 312 p.
- Mayr, E., 1963, Animal Species and Evolution: Cambridge, Belknap, 811 p.
- McGhee, G.R., Jr., 1984, Tempo and mode of the Frasnian-Famennian biotic crisis: Geological Society of America Abstracts with Programs, v. 16, no. 6, p. 49.
- McGhee, G.R., Jr., 1996, The Late Devonian Mass Extinction; The Frasnian/Famennian Crisis: New York, Columbia University Press, 303 p.
- McGhee, G.R., Jr., 2005, Modelling Late Devonian extinction hypotheses, in Over, D.J., Morrow, J.R., and Wignall, P.B., eds., Understanding Late Devonian and Permian-Triassic Biotic and Climatic Events: Towards an Integrated Approach: Amsterdam, Elsevier, p. 37–50.
- McGhee, G.R., Jr., Sheehan, P.M., Bottjer, D.J., and Droser, M.L., 2004, Ecological ranking of Phanerozoic biodiversity crises, ecological and taxonomic severities are decoupled: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 211, p. 289–297.
- Racki, G., 2005, Toward understanding Late Devonian extinction hypotheses: Few answers, many questions, in Over, D.J., Morrow, J.R., and Wignall, P.B., eds., Understanding Late Devonian and Permian-Triassic Biotic and Climatic Events: Towards an Integrated Approach: Amsterdam, Elsevier, p. 5–36.
- Raup, D.M., and Sepkoski, J.J., Jr., 1982, Mass extinctions in the marine fossil record: Science, v. 215, p. 1501–1503.
- Rode, A.L., 2004, Phylogenetic revision of the Devonian bivalve, Leptodesma (Leiopteria): Yale University Postilla, v. 229, p. 1–26.
- Rode, A.L., and Lieberman, B.S., 2002, Phylogenetic and biogeographic analysis of Devonian phyllocarid crustaceans: Journal of Paleontology, v. 76, p. 269–284.
- Rode, A.L., and Lieberman, B.S., 2004, Using GIS to unlock the interactions between biogeography, environment, and evolution in Middle and Late Devonian brachiopods and bivalves: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 211, p. 345–359.
- Smith, A.B., 1994, Systematics and the Fossil Record: Documenting Evolutionary Patterns: Oxford, Blackwell Scientific, 223 p.
- Stanley, S.M., 1979, Macroevolution: Pattern and Process: San Francisco, W.H. Freeman and Co., 332 p.
- Stigall, A.L., 2010a, Invasive species and biodiversity decline: Testing the link in the Late Devonian: PLoS ONE, v. 5, no. 12, e15584, http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0015584 (last accessed 7 Nov. 2011).
- Stigall, A.L., 2010b, Using GIS to assess the biogeographic impact of species invasions on native brachiopods during the Richmondian Invasion in the Type-Cincinnatian (Late Ordovician, Cincinnati region): Palaeontologia Electronica 13, 5A, http://palaeo-electronica.org/2010_1/207/index.html (last accessed 4 Nov. 2011).
- Stigall Rode, A.L., 2005, Systematic revision of the Devonian brachiopods Schizophoria (Schizophoria) and “Schuchertella” from North America: Journal of Systematic Palaeontology, v. 3, p. 133–167.
- Stigall Rode, A.L., and Lieberman, B.S., 2005, Using environmental niche modelling to study the Late Devonian biodiversity crisis, in Over, D.J., Morrow, J.R., and Wignall, P.B., eds., Understanding Late Devonian and Permian-Triassic Biotic and Climatic Events: Towards an Integrated Approach: Amsterdam, Elsevier, p. 93–180.
- Thuiller, W., 2007, Biodiversity—Climate change and the ecologist: Nature, v. 448, p. 550–552.
- van Geldern, R., Joachimski, M.M., Day, J., Jansen, U., Alvarez, F., Yolkin, E.A., and Ma, X.P., 2006, Carbon, oxygen and strontium isotope records of Devonian brachiopod shell calcite: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 240, p. 47–67.
- Vermeij, G.J., 2005, Invasion as expectation: A historical fact of life, in Sax, D.F., Stachowicz, J.J., and Gaines, S.D., eds., Species Invasions: Insights into Ecology, Evolution and Biogeography: Sunderland, Massachusetts, Sinauer, p. 315–339.
- ver Straeten, C.A., Brett, C.E., and Sageman, B.B., 2011, Mudrock sequence stratigraphy: A multi-proxy (sedimentological, paleobiological, geochemical) approach, Devonian Appalachian Basin: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 304, p. 54–73.
- Vrba, E.S., 1987, Ecology in relation to speciation rates: Some case histories of Miocene-Recent mammal clades: Evolutionary Ecology, v. 1, p. 283–300.
- Wiley, E.O., and Mayden, R.L., 1985, Species and speciation in phylogenetic systematics, with examples from North American fish fauna: Annals of the Missouri Botanical Garden, v. 72, p. 596–635.

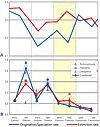 Figure 1
Figure 1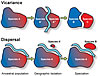 Figure 2
Figure 2 Figure 3
Figure 3 Table 1
Table 1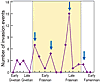 Figure 5
Figure 5